Will 2024 be a leap year for Liquid Biopsy?
Liquid biopsy is red hot - investor expectations are soaring in expectation of upcoming approvals and continually growing number of trials. Time for pharma to intensify preparation?
I: What is liquid biopsy?
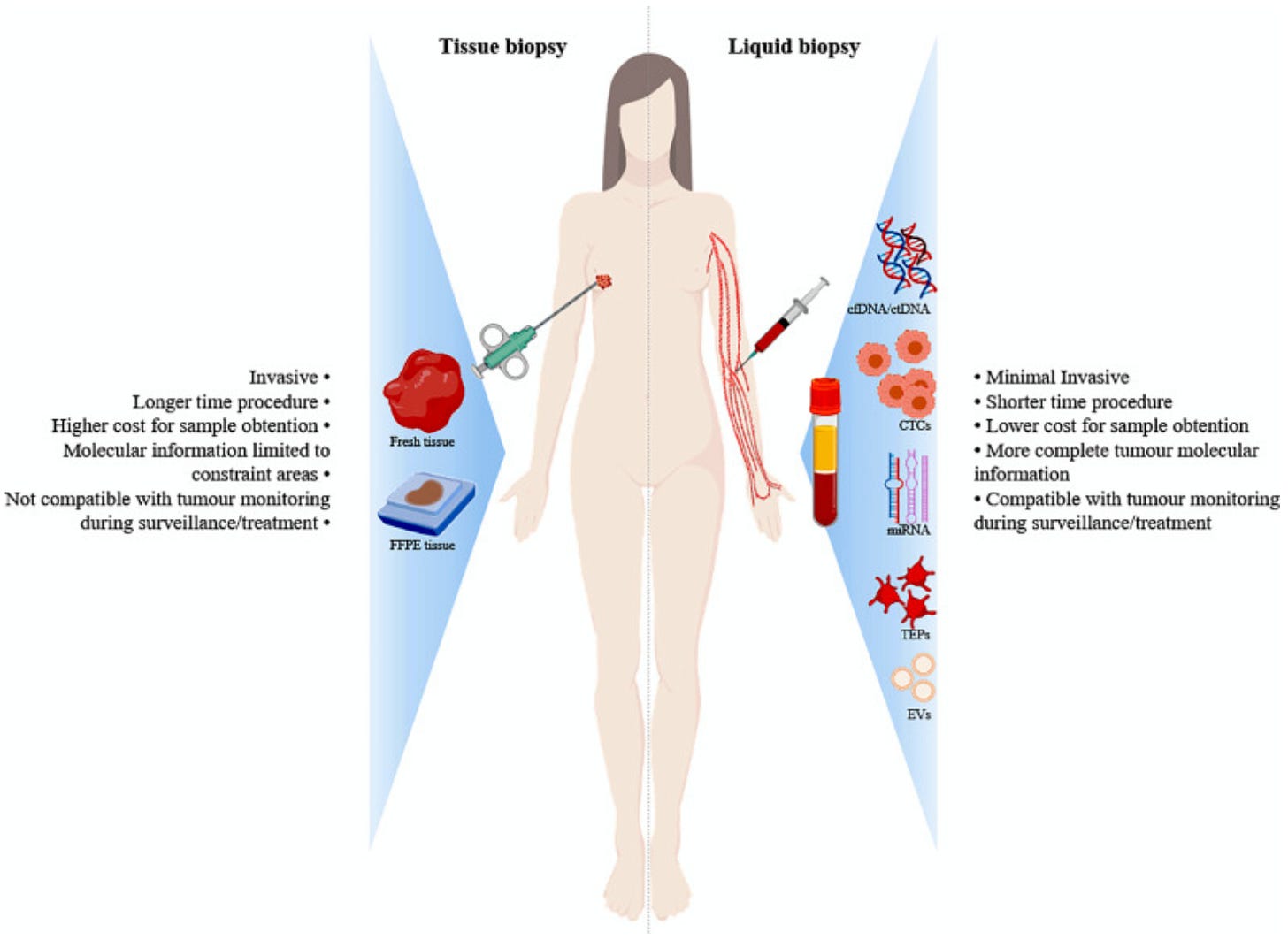
Liquid biopsy is a blood test that measures pieces of tumours in a patient’s blood - as opposed to taking a piece of the tumour itself in tissue biopsy.
There are four major use cases for liquid biopsy:
Therapy selection, where biomarker profiling of the tumour enables using a more targeted therapy
Minimal residual disease (MRD) measurement, where cancer levels are measured to identify patients with where a “watch and wait” approach could be appropriate, understand patient response to a therapy or to watch for cancer recurrence during remission
Cancer screening to identify malignancies in asymptomatic individuals
A variety of non-oncology settings, e.g., prenatal screening for genomic anomalies
II: Is it likely to become mainstream?
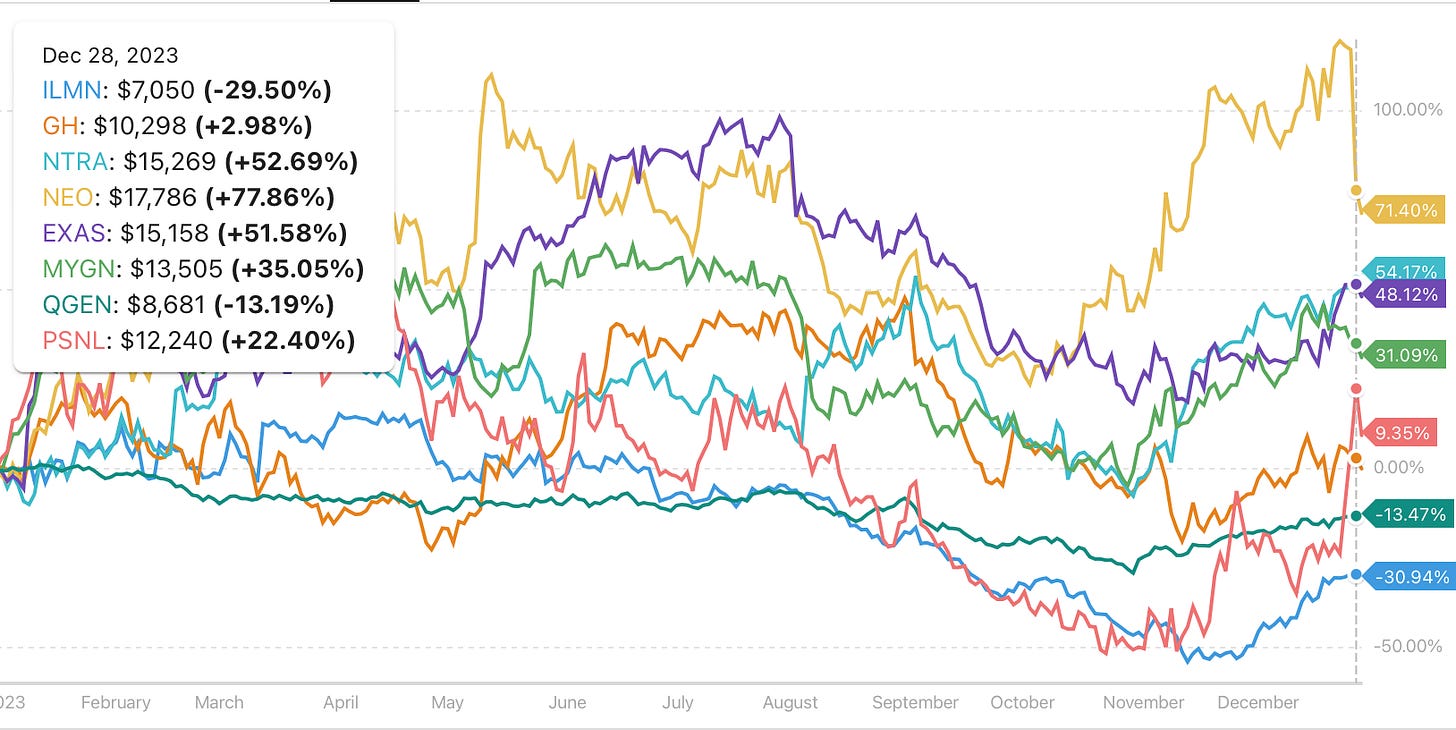
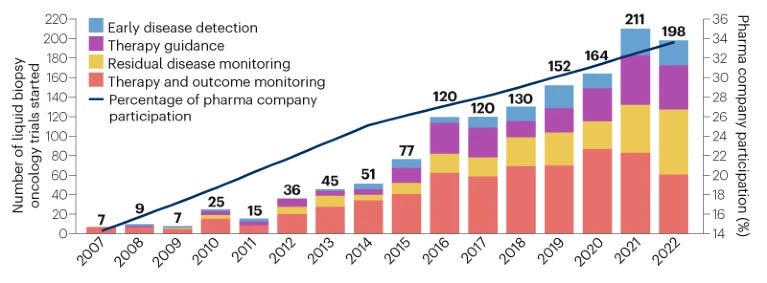
Funds from financial investors are pouring into companies like Guardant Health, Natera and Illumina, reflecting a bullish sentiment around the liquid biopsy market. This is fueled by several factors:
Increasing support from regulators, most importantly demonstrated by FDA’s 2022 guidance document “Use of Circulating Tumor DNA for Early-Stage Solid Tumor Drug Development”
Increasing number of oncology trials using liquid biopsy
Clear advantages of liquid biopsy over other diagnostics methods (especially low invasiveness, repeatability, speed of results, ability to biopsy tumours where tissue is not reachable)
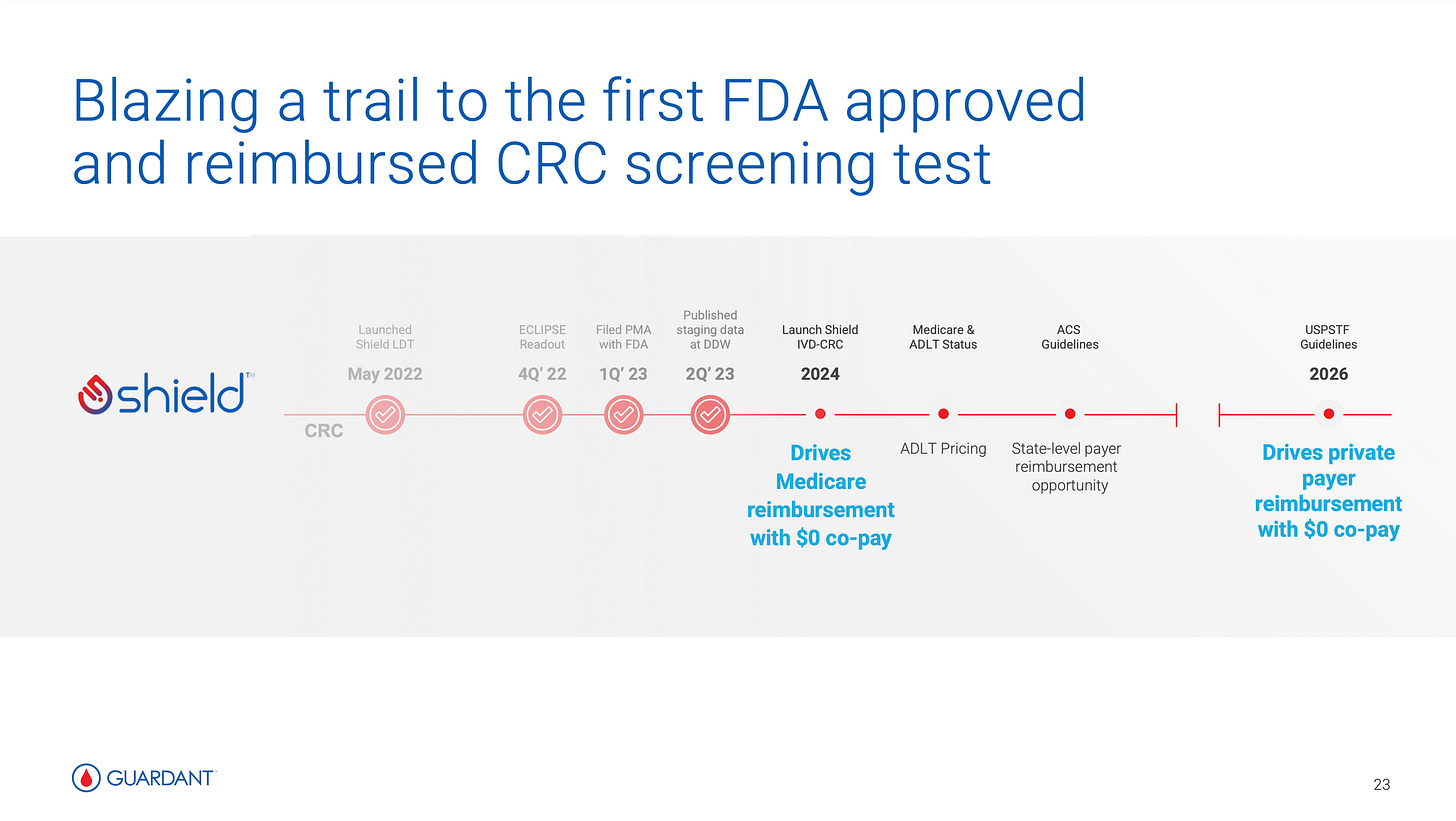
Upcoming launch of multiple key products, most importantly Guardant Health’s Shield (reimbursed early stage colorectal cancer screening test) which could potentially be a watershed moment for wide-spread screening
III: What could this mean for biopharma?
Liquid biopsy going mainstream may have several implications that biopharma companies are already starting to prepare for.
Identify opportunities to benefit from liquid biopsy - e.g., improve treatment and trial experience, accelerate trial execution (surrogate markers enable earlier go/no-go decisions)
Understand potential new patient pathways - especially as a result of more wide-spread screening (reimbursed and self-paid; single-target and multi-cancer).
Prepare for changing patient expectations - e.g., on transparency of prognosis (which could be inferred from liquid biopsy tests)
Consider partnering with select liquid biopsy companies to build competitive advantage, e.g., in developing targeted oncology products
Read more/ sources:
Liquid Biopsy: A Distinctive Approach to the Diagnosis and Prognosis of Cancer
Liquid biopsy: from concept to clinical application - Scientific Reports
Liquid biopsy epigenomic profiling for cancer subtyping - Nature Medicine
The Expanding Potential of Liquid Biopsy to Detect and Monitor Cancer
Challenges and achievements of liquid biopsy technologies employed in early breast cancer
Investor presentations of Guardant Health, Illumina